The pH of a soil influences the entire soil chemical environment and fundamentally determines nutrient availability, fertilizer response, and soil biology. In general, a neutral pH is considered adequate for most turfgrass needs; however, slightly more acidic pH can allow for increased levels of metal ions to become soluble and is often favored as a means of increasing the competitiveness of creeping bentgrass and fine fescue over annual bluegrass.
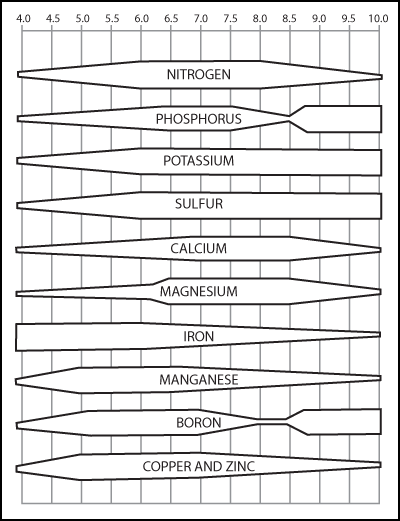 Relative soil nutrient availability as influenced by pH. Source: Virginia Turfgrass Council.
Relative soil nutrient availability as influenced by pH. Source: Virginia Turfgrass Council.Soil pH can be manipulated with a variety of fertilizer sources such as ammonia sources of nitrogen that have a slight acidifying effect as ammonium is processed by microbes. In addition, various types of liming materials such as calcium carbonate or dolomitic (higher Mg) lime supply nutrients can raise the soil the pH. Salts can also raise pH.
Efforts have been made to reduce soil pH with elemental sulfur to address calcareous soil issues with pH in excess of 7.3. Due to the high buffering capacity (the ability of soils where calcium or magnesium is a parent material to resist a pH change) of soils with pH greater than 7.3, the use of elemental sulfur results in little change. This result is especially true for limestone based soils in great lakes region of NY.
Soil pH profoundly influences phosphorus (P) availability and can influence movement on and through the soil profile. Soil available P or P fertilizer added is either fixed by adsorption to soil particles or retained without precipitating into secondary P minerals. The amount of fixation and retention depends on a wide array of factors, pH being one of the most significant. Precipitation increases as iron or aluminum precipitates at acid pH or as calcium precipitate at alkaline pH. The pH equilibrium between these precipitation extremes is between 6.0 and 7.0
